Baeyer Strain Theory
Cyclopropane reacts with chlorine and bromine in dark to form addition products (ring opening reaction). Cyclobutane and higher cycloalkanes do not give this ring opening reaction at ordinary conditions. This can be explained by the basis of angle strain theory or Baeyer Strain Theory.
Assumptions of Baeyer Strain Theory
All ring systems are planar.
Deviation from normal tetrahedral angle results in angle strain that makes the cycloalkanes to become unstable.
The large ring systems involve negative strain hence do not exist.
Besides torsional strain and steric strain, the conformations of cycloalkanes are also affected by angle strain.
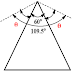
Angle Strain in Cyclopropane
- In cyclopropane, C-C-C bond angle is 60°.
- This revealed that normal tetrahedral angle of 109.5° between any two bonds is compressed to 60°. Hence it has more angle strain.
Calculation of Angle strain
θ + 60 + θ = 109.5
2θ = 109.5 – 60
θ = 49.5 / 2
θ = 24.5o
Hence, cyclopropane has an angle strain of 24.5 o.
Angle Strain in cyclobutane
- In cyclobutane, C-C-C bond angle is 90°.
- This revealed that normal tetrahedral angle of 109.5° between any two bonds is compressed to 90°.
Calculation of Angle strain
θ + 90 + θ = 109.5
2θ = 109.5 – 90
θ = 19.5 / 2
θ = 9.75o
Hence, cyclobutane has an angle strain of 9.75 o.
Angle Strain in cyclopentane
- In cyclopentane, C-C-C bond angle is 108°.
- This revealed that normal tetrahedral angle of 109.5° between any two bonds is compressed to 108°.
Calculation of Angle strain
θ + 108 + θ = 109.5
2θ = 109.5 – 108
θ = 1.5 / 2
θ = 0.75o
Hence, cyclopentane has an angle strain of 0.75 o.
- In cyclohexane, C-C-C bond angle is 120°.
- This revealed that normal tetrahedral angle of 109.5° between any two bonds is expanded to 120°.
Calculation of Angle strain
θ + 120 + θ = 109.5
2θ = 109.5 – 120
θ = -10.5 / 2
θ = - 5.25o (Expansion)
Hence, cyclohexane has an angle strain of -5.25o.
According to Baeyer’s Strain theory, cyclopentane is the most stable cycloalkane than cyclopropane, cyclobutane and cyclohexane due to less angle strain. The order of stability follows
Cyclopropane < cyclobutene < cyclohexane < cyclopentane
Cycloalkane | Actual C-C-C bond angle | Angle Strain |
Cyclopropane | 60 | 24.5 |
Cyclobutane | 90 | 9.75 |
Cyclopentane | 108 | 0.75 |
Cyclohexane | 120 | -5.25 |
Limitations of Baeyer Strain Theory
- According to enthalpy of combustion, cyclohexane is more stable than cyclopentane. Baeyer Theory does not explain this behavior. The correct order of stability of cycloalkanes are as follows. Cyclopropane < cyclobutene < cyclopentane < cyclohexane
- Baeyer assumed all the cycloalkanes as planar molecules.
- Actually, except cyclopropane, all other cycloalkanes are not planar.






No comments:
Post a Comment