Conformational Analysis of Ethane and Butane
• Different spatial arrangement of molecules that are generated by rotation about single bonds.
• Conformational analysis is the study of the effect of rotation on the properties of a molecule.
Torsional strain
It exists when neighboring carbon posses hydrogens that overlap in space (eclipse). The torsional strain arises when a molecule is rotated around a bond.
Steric strain
It is the repulsion between two atoms or groups of atoms when the distance between them is decreased. Steric strain is formed when two or more bulky groups get close to each other.
Conformation of Ethane
• Although there are SEVEN sigma bonds in the ethane molecule, rotation about the six carbon-hydrogen bonds does not result in any change in the shape of the molecule.
• Rotation about the carbon-carbon bond results in many different possible molecular conformations.
• Types of conformations are
§ Eclipsed conformation
§ Staggered conformation
§ Skew conformation (infinite intermediate conformation)
Conformations of butane
There are THREE C-C sigma bonds in the butane molecule, rotation about the C2-C3 carbon results in many different possible molecular conformations.
Types of conformations are
- Fully Eclipsed conformation (Syn)
- Eclipsed conformation
- Gauche conformation
- Fully staggered conformation (anti)
- Skew conformation (infinite intermediate conformation)
Fully Eclipsed Conformation (Syn Conformation)
This conformation arises when the dihedral angle between two CH3 group is 0 degree leading to maximum torsional and steric strain. Hence this conformation is unstable compared to all other conformations.
Gauche Conformation
As rotation around the C(2)–C(3) bond occurs by 60 degree from fully eclipsed conformations, staggered conformation is reached in which dihedral angle between Me–Me groups are 60 degree. Due to less torsional and steric strain, it is relatively stable than fully eclipsed conformation.
Eclipsed Conformation
As rotation around the C(2)–C(3) bond occurs from gauche conformation by 60 degree, another eclipsed conformation is reached in which there are two Me–H interactions and one H–H interaction. Due to torsional and steric strain, it is relatively unstable than gauche conformation.
Anti Staggered conformation
The lowest-energy arrangement, called the antiperiplanar (or anti) conformation, is the one in which the two large methyl groups are as far apart as possible. Hence It experiences minimum torsional and steric strain
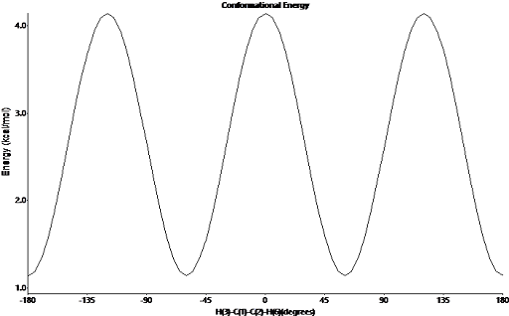
The potential energy diagram of butane conformers is as follows. The stability of conformations of butane follows the order ;
anti-staggered > gauche > Eclipsed > Fully eclipsed









No comments:
Post a Comment